A personal message from Rick Bollinger.
|
I would like you to (1) take a survey over the internet, (2) schedule a 40 minute interview, and later (3) join me in a Zoom session.
What's in your history files? They tell your story, right? What would you have them say?
My name is Rick Bollinger. I am Founder and CEO of both Menlo Park Associates and Menlo Park Software. I hold an MSE in computer engineering and a BSE in industrial engineering (cum laude). Both from the University of Michigan.
|
Each step in this process contains the same questions. Some can leisurely be done first in the survey, and the more complex ones done in a 40 minute Zoom interview.
Please join my Customer Discovery campaign. Interview to explore your most pressing problems TODAY and how you deal with them.
I've managed many projects in several industries, including medical device manufacture.
|
Please go to MenloParkSoftware.com to
(1) take a leisurely survey,
(2) schedule an interview and
(3) join a 40 minute Zoom session
Free Diagnostic Services
|
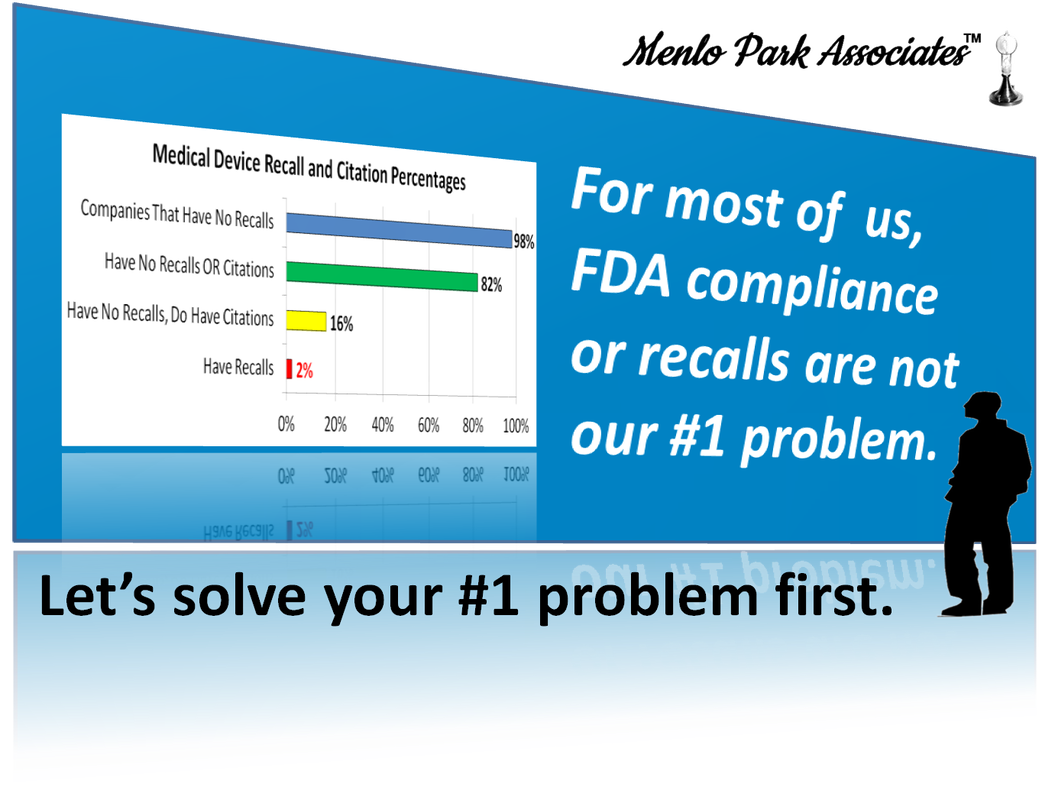
I am researching problems in the medical device industry. Common or chronic problems suggest solutions could be industry-wide. You may find yourself at the beginning of difficult changes. We can start with your biggest problem that is pressing today. The Discovery Campaign is FREE!
|
Menlo Park Associates owns Menlo Park Software. This structure was intended to provide support for Menlo Park Software products and consulting for its introduction into methods solving complex problems. That's why interviews and Dx services are currently FREE.
|
What's in Your History Files? ™
|
Richard L. Bollinger
618 Fifth Street Ann Arbor, MI 48103 |